Can the Atomic Mass of an Element Vary
Naturally occurring boron consist of two isotopes with accurately known masses 10 B 100129 amu and 11B 110931 amu. Adding or losing neutrons will change the atomic mass without forming a different elemen D.

What Is Atomic Mass Definition And Examples Video Lesson Transcript Study Com
The atomic mass is the total number of nucleons neutrons and protons in an atom.
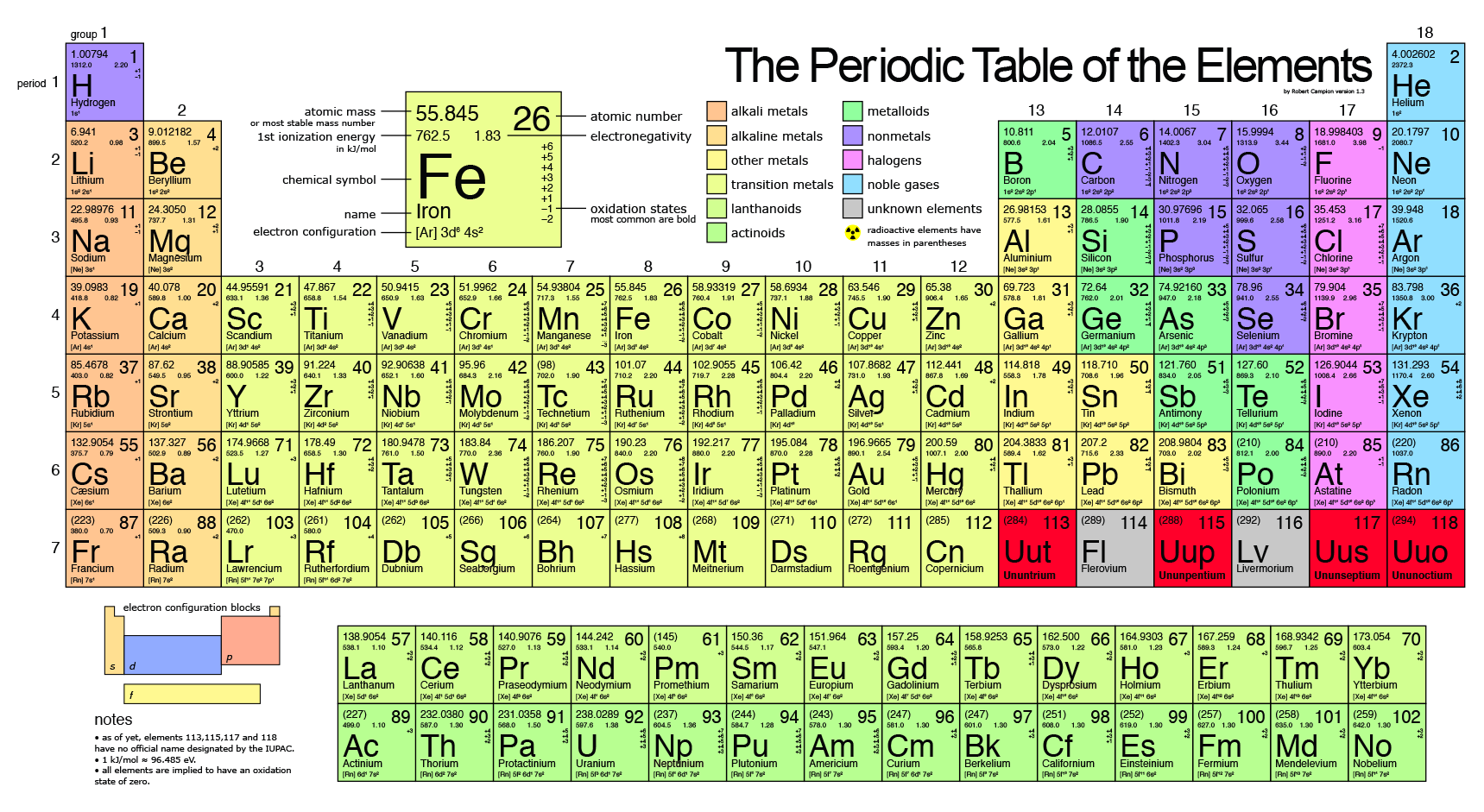
. Carbon hydrogen nitrogen oxygen Can the atomic mass of an element vary. The weight of an electron is almost negligible. The average mass of an element remains essentially staple as an average of the common isotopes.
Thus the atomic mass of an atom is almost the same as its mass number. Can the atomic mass of an element vary. The mass of an individual atom will not change unless the atom undergoes a nuclear change.
Adding or losing neutrons will change the atomic mass without forming a different element. The mass varies within an element based on the number of neutrons. The atomic mass of an element can vary.
There are different forms of some elements which contain the same number of protons but which have different. Adding or losing neutrons will change the atomic mass without forming a different element. Therefore an elements atomic number will never change.
The actual atomic mass of boron can vary grom 10807 to 10819 depending on whether the mineral source is from Turkey or the United States. How can the atomic mass of an element vary. Atomic mass however can change and we call these isotopes.
This is called the relative isotopic mass. Each variety of an atom within an element based on a different number of neutrons is called an isotope of that element. While the number of protons remains the same in all atoms of an element the number of neutrons can vary.
20 Can the atomic mass of an element vary. Isotopes are the same element with different atomic masses. However the mass number of a sample of an element can and will change over time as the radioactive isotopes undergo nuclear changes.
Can the atomic mass of an element vary. Adding or losing electrons will substantially change the atomic mass. If it changes at all then you have formed a different element.
Adding or losing protons will change the atomic mass without forming a different element. A No it is fixed. 22 Can the atomic mass of an element vary.
The properties of elements depend upon the number of electrons present in the valence shell which are related to atomic numberthus the properties of different elements can be compared if we know their atomic numberson the other hand atomic mass can in no way determine the chemical proper-ties of elements because it does not vary regularly. Adding or losing neutrons will change the atomic mass without forming a different element. The average mass of an element remains essentially staple as an average of the common isotopes.
Adding or losing electrons will substantially change the atomic mass B. An elements atomic number will never change that is because the atomic number is its identity The atomic number is the number of protons that is contained in the nucleus if you add a proton you change the element. Which four of these twenty-five elements make up approximately 96 percent of living matter.
Adding or losing electrons will substantially change the atomic mass. Together the number of protons and the number of neutrons determine an elements mass number. If it changes at all then you have formed a different element.
A No it is fixed. Otherwise a new element will be formed. The main reason the atomic number is important is because its how you identify the element of an atom.
A No it is fixed. Adding or losing protons will change the atomic mass without forming a different element C. History of Atomic Number.
We often refer to an isotope by stating the elements name followed by its mass number for example magnesium-24. Most elements found in nature exist in isotopic forms. Can the atomic mass of individual atoms of an element vary.
How many electrons are involved in a double covalent bond. The average atomic masses of some elements may vary depending upon souces of their ores. Thus atoms of the same element can have different mass numbers and these are called isotopes.
Adding or losing protons will change the atomic mass without forming a different element. - Answers The mass of an atom will always remain constant but various isotopes of the same element may have differing atomic masses. Adding or losing electrons will substantially change the atomic mass.
Mass of molecules can be determined by adding the average atomic mass of each atom in the molecule. In contrast the number of neutrons for a given element can vary. Mass number protons neutrons.
Can the atomic mass of an element vary Yes I do youre losing neutrons will change the atomic mass without forming a different element A covalent chemical bond is one in which Outershell electrons of two Adams are shared so as to satisfactorily feel the respective orbitals. However the mass number of a sample of an element can and will change over time as the radioactive isotopes undergo nuclear changes. Atomic Mass of Elements.
The mass of an individual atom will not change unless the atom undergoes a nuclear change. Atomic mass however can. The Atomic mass of some elements is tabulated below.
Why is knowing the atomic mass of an element important. Can the atomic mass of an element vary. Forms of the same atom that differ only in their number of neutrons are called isotopes.
All naturally occurring magnesium atoms contain 7899 magnesium-24 1000 magnesium-25 and 1101 magnesium-26. Adding or losing neutrons will change the atomic mass without forming a different element. The atomic number is the number of protons that is contained in the nucleus if you add a proton you change the element.
The atomic masses of elements vary from 1008 amu for hydrogen up to 250 amu for elements which have a very high atomic number. Adding or losing protons will change the atomic mass without forming a. The atomic mass is the sum of the protons and neutrons in the nucleus in an isotope you have more neutrons than you.

Relative Atomic Mass A Question Science By Degrees
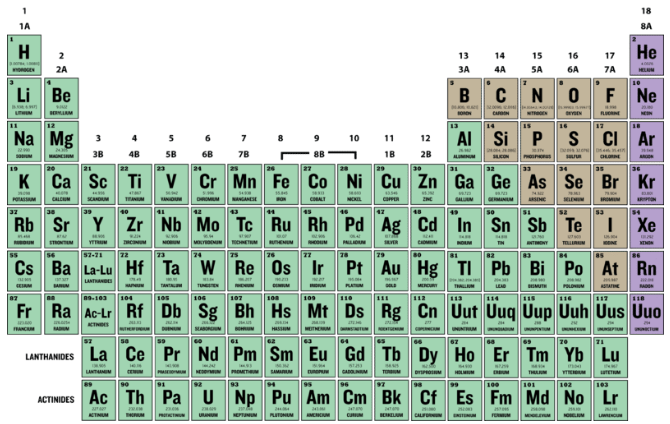
2 1 Isotopes And Atomic Mass Chemistry Libretexts

3 4 Atomic Mass And Atomic Number Chemistry Libretexts Atomic Number Periodic Table Mass Number

Defining How To Calculate Relative Atomic Mass Of Element Relative Isotopic Mass Definition Gcse Chemistry Calculations Igcse O Level Revision Notes

What Happens To The Atomic Mass As You Go Down Each Group Family Socratic

1 9 Atomic Mass The Average Mass Of An Element S Atoms Chemistry Libretexts

Atomic Number Atomic Mass And Isotopes Article Khan Academy

Trends In Modern Periodic Table Class 10 Periodic Classification Of Elements Periodic Table Element Symbols Element Chemistry

0 Response to "Can the Atomic Mass of an Element Vary"
Post a Comment